Compliant and Audit-Ready
The LANEXO® inventory manager uses validated software that supports laboratory compliance with FDA 21 CFR Part 11 and EU GMP Annex 11, thanks to several essential features, including:
- User authentication requirements
- A unique digital signature for each user
- Time-stamped audit trails
Data Traceability
In capturing electronic data via simple scanning of RFID labels on consumables, LANEXO®ensures reliability, integrity and traceability by:
- Improving the quality of the data for reagents, standards, samples and lab-prepared mixtures and intermediates
- Eliminating the risk of human error during data transcription
- Improving the searchability of digital records
Documentation
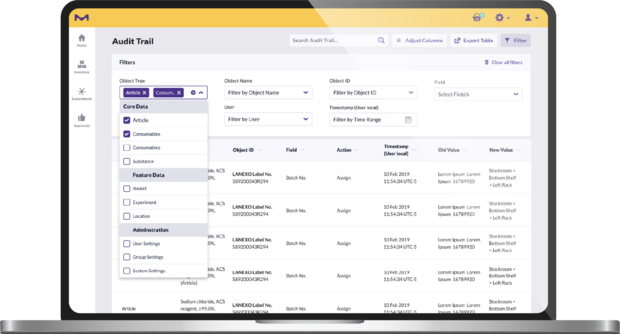
Each consumable registered into the system is matched to readily available, easily searchable digital documentation, including a complete time-stamped audit report, documenting:
- Initial registration
- Ownership
- Opening date and respectively calculated expiration date
- Use in the creation of a mixture or intermediate, which can be tracked back to educts
- Use in an experimental workflow
- Disposal
Any actions performed in the app are captured in the audit trail and can be easily exported. This complete traceability makes audit trails more reliable and labs more audit ready. It is also easier for users to report hazardous materials and monitor chemical consumption. This feature includes all events related to location, consumables and users. Each consumable has a time-stamped event source audit, making its lifecycle traceable in the audit trail.
Experimental Reporting
The high-quality data captured by LANEXO® maximizes ease and accuracy in inventory management, experimental reporting and safety compliance.
- Specification and identity checks along experimental workflows are simplified.
- Lab personnel can easily and quickly identify reagents, samples, intermediates and mixtures by scanning RFID labels, helping to avoid errors.
- Time-stamped information on expiry dates, volume tracking and data traceability greatly simplifies quality checks of input/output consumables used in experiments.
User Compliance
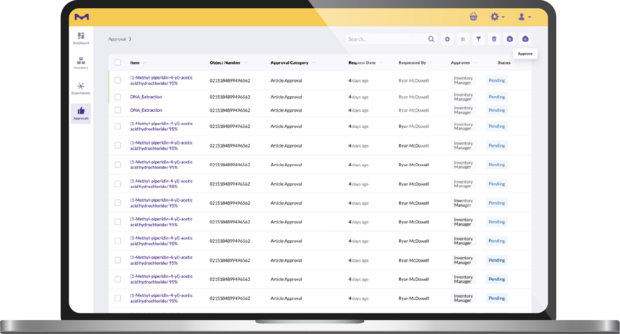
Approval & user management: Set user permissions according to your lab’s setup and approval processes.
What's included